Synthesis, in vitro and in vivo evaluation of new hybrids of millepachine and phenstatin as potent tubulin polymerization inhibitors†
Abstract
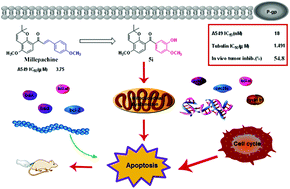
In this paper, a series of millepachine derivatives were synthesized and evaluated as tubulin polymerization inhibitors. The optimal compound 5i, (3-hydroxy-4-methoxyphenyl)(5-methoxy-2,2-dimethyl-2H-chromen-8-yl)methanone, displayed the highest cytotoxicity toward a series of cancer cells (ranging from 18 to 45 nM of IC50). Further investigation revealed that 5i significantly repressed the multidrug resistant cells (A549/CDDP, A2780/TAX) and had little cytotoxicity towards human normal cells (HLF, BJ). Cellular mechanism studies demonstrated that 5i induced G2/M phase arrest and apoptosis, which was associated with the collapse of the mitochondrial membrane potential (MMP). Additionally, western blot analysis showed that 5i could change the levels of cell cycle-related proteins (e.g. Cyclin B1, Cdc25c, Cdc2) and some apoptosis-related proteins (e.g. Bax, Bad, Bcl-2, Bcl-xl). Finally, 5i effectively inhibited the growth of xenograft tumours of A549 cells in nude mice.


 Please wait while we load your content...
Please wait while we load your content...