Diels–Alder trapping of in situ generated dienes from 3,4-dihydro-2H-pyran with p-quinone catalysed by p-toluenesulfonic acid†
Abstract
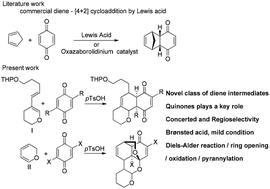
This comprehensive study portrays that p-toluenesulfonic acid is a more efficient catalyst for the reaction between p-quinones and 3,4-dihydro-2H-pyran, than the Lewis acids. The products were accomplished by the Diels–Alder cycloaddition reaction and their mechanistic pathways have been formulated. The impact of C2 and C2,5 substituents of the p-quinones on the cycloaddition reaction has been explored. Remarkably, it is the first report to explore this kind of in situ generated diene for the Diels–Alder cycloaddition reaction.


 Please wait while we load your content...
Please wait while we load your content...