Ferromagnetic ordering and slow magnetic relaxation observed in a triple-bridged azido-Cu(ii) chain compound with mixed carboxylate/ethanol linkers†
Abstract
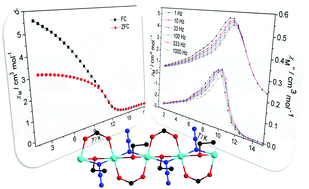
Solvothermal reactions of copper salts with NaN3 and carboxylate led to the formation of a new magnetic compound, [Cu(3-fba)(N3)(C2H5OH)]n (3-fba = 3-fluorobenzoic acid) (1). Single crystal X-ray analysis indicates that only one crystallographically independent Cu(II) ion in the asymmetric unit of 1 displays stretched octahedral geometry in which two azido N atoms and two carboxylic O atoms locate in the equatorial square, while two ethanol O atoms occupy the apical positions, yielding a 1D Cu(II) chain with an alternating triple-bridge of EO-azido, syn,syn-carboxylate, and μ2-ethanol. Magnetic measurements reveal that the resulting compound is composed of well-isolated 1D Cu(II) chains with strong intrachain ferromagnetic interactions. The dominant ferromagnetic couplings between neighbouring Cu(II) ions occurring in the compound (J = 59.74 cm−1) are due to the counter-complementarity of the multiple superexchange pathways, contributing to inspiring plots of ferromagnetic order (Tc = 11.0 K) and slow magnetic relaxation that are rarely observed in most reported azido-Cu(II) architectures. Heat-capacity experiments further emphasize the characteristic long-range ferromagnetic ordering in 1. Moreover, density functional theory (DFT) calculations (using different methods and basis sets) have been performed on the compound to obtain the theoretical interpretation of the magnetic behaviors.


 Please wait while we load your content...
Please wait while we load your content...