Abstract
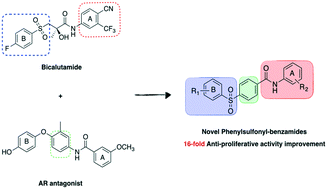
Prostate cancer is a major cause of male death worldwide and the identification of new efficient treatments is constantly needed. Different non-steroidal androgen receptor antagonists are approved also in the case of castration-resistant cancer forms. Using a rational approach and molecular modelling studies to modify the structure of antiandrogen drug bicalutamide, a new series of phenylsulfonyl-benzamide derivatives was designed and synthesised. Their antiproliferative activities were evaluated in four different human prostate cancer cell lines and several new compounds showed significantly improved IC50 values in the low μM range. The cytotoxicity profile was also evaluated for the novel structures in the HEK293 cell line.


 Please wait while we load your content...
Please wait while we load your content...