Abnormal site occupancy and high performance in warm WLEDs of a novel red phosphor, NaHF2:Mn4+, synthesized at room temperature†
Abstract
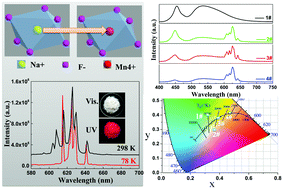
A novel red phosphor, NaHF2:Mn4+ (NHF:Mn), was obtained via substituting Na+ located at the center of the octahedron coordinated with six F− ions with Mn4+ in the host lattice of NHF. The phase purity and the exact composition of the obtained NHF:Mn were confirmed by X-ray powder diffraction (XRD), Rietveld refinement, energy dispersive spectroscopy (EDS), transmission electron microscopy (TEM), and infrared (IR) spectroscopy, respectively. The luminescence intensity of NHF:Mn was enhanced by optimizing the synthetic conditions. A series of warm white light-emitting diodes (WLEDs) with a color rendering index (CRI) higher than 88.0 and correlated color temperatures (CCT) between 3146 and 5172 K were obtained by encapsulating the as-prepared red phosphor NHF:Mn with the yellow one Y3Al5O12:Ce3+ (YAG:Ce) on blue chips. The advantage of the synthetic strategy to obtain NHF:Mn can be extended to develop novel Mn4+ doped red phosphors via substituting for central ions with unequal electric charge in the centers of octahedra.


 Please wait while we load your content...
Please wait while we load your content...