Deactivation of the coordinating ability of the iminophosphorane group by the effect of ortho-carborane†
Abstract
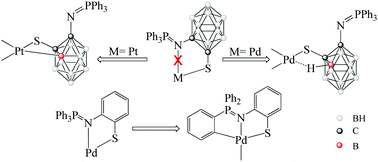
The C-carboranyl iminophosphorane compounds derived from triphenylphosphine (I1) and o-(methylthiophenyl)-diphenylphosphine (I2) were obtained and structurally characterized. Functionalization with sulfur on the other cage carbon atom gave the corresponding disulfides (L1L1 and L2L2). In the case of I1 it was also possible to isolate the trisulfide (L1SL1) and the thiol (L1H), which is not in the expected zwitterionic form, showing that the carborane group reduces the basicity of the nitrogen atom. The disulfides were made to react with the zero-valent metal precursors [M(PPh3)4], where M = Pd, Pt. The structural analysis of the final complexes revealed the non-coordinating nature of the nitrogen atom. The isolated platinum complexes (PtL1 and PtL2) show metalation on an adjacent boron atom of the carborane cage while the palladium complexes (PdL1 and PtL2) do not complete the BH activation and are isolated as pseudo-agostic complexes. The weak pseudo-agostic interaction can be displaced by incoming ligands. Thus, reaction of PdL1 with dppm and dppe diphosphines leads to the isolation of the cis-bis(thiolates) [Pd(L1)2(dppm)] (PdL1dppm) and [Pd(L1)2(dppe)] (PdL1dppe). The non-carboranyl disulfide analogue (L3L3) was prepared and made to react with the zero-valent palladium precursor. The structure of the non-carboranyl palladium analogue (PdL3) shows the coordinating ability of the nitrogen donor atom, proving the deactivating effect of the C-carboranyl group.


 Please wait while we load your content...
Please wait while we load your content...