Organometallic gold(iii) complexes with hybrid SNS-donating thiosemicarbazone ligands: cytotoxicity and anti-Trypanosoma cruzi activity†
Abstract
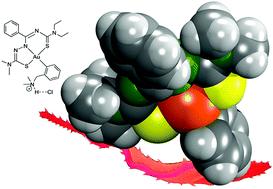
Stable organogold(III) compounds of the composition [AuIII(Hdamp)(L1)]Cl are formed from reactions of [AuCl2(damp)] with H2L1 (damp− = dimethylaminomethylphenyl; H2L1 = N′-(diethylcarbamothioyl)benzimidothiosemicarbazides). The cationic complexes can be neutralized by reactions with weak bases under the formation of [AuIII(damp)(L1)] compounds. The structures of the products show interesting features like relatively short Au⋯H contacts between the methylene protons of the Hdamp ligand and the gold(III) ions. Preliminary biological studies on the uncoordinated compounds H2L1 and their gold complexes indicate considerable cytotoxicity for the [AuIII(Hdamp)(L1)]Cl complexes against MCF-7 cells. The in vitro trypanocidal activity was evaluated against the intracellular form of Trypanosoma cruzi. The organometallic complexes display a remarkable activity, which is dependent on the alkyl substituents of the thiosemicarbazone building blocks of the ligands. One representative of the cationic [AuIII(Hdamp)(L1)]Cl complexes, where H2L1 contains a dimethylthiosemicarbazide building block, shows a trypanocidal activity against the intracellular amastigote form in the same order of magnitude as that of the standard drug benznidazole. Furthermore, no appreciable toxicity to mice spleen cells is observed for this compound resulting in a therapeutic index of about 30, which strongly recommends it as a promising candidate for the development of a future antiparasitic drug.


 Please wait while we load your content...
Please wait while we load your content...