Composition-dependent charge transfer and phase separation in the V1−xRexO2 solid solution
Abstract
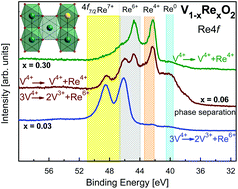
The substitution of vanadium in vanadium dioxide VO2 influences the critical temperatures of structural and metal-to-insulator transitions in different ways depending on the valence of the dopant. Rhenium adopts valence states between +4 and +7 in an octahedral oxygen surrounding and is particularly interesting in this context. Structural investigation of V1−xRexO2 solid solutions (0.01 ≤ x ≤ 0.30) between 80 and 1200 K using synchrotron X-ray powder diffraction revealed only two polymorphs that resemble VO2: the low-temperature monoclinic MoO2-type form (space group P21/c), and the tetragonal rutile-like form (space group P42/mnm). However, for compositions with 0.03 < x ≤ 0.15 a phase separation in the solid solution was observed below 1000 K upon cooling down from 1200 K, giving rise to two isostructural phases with slightly different lattice parameters. This is reflected in the appearance of two metal-to-insulator transition temperatures detected by magnetization and specific heat measurements. Comprehensive X-ray photoelectron spectroscopy studies showed that an increased amount of Re leads to a change in the Re valence state from solely Re6+ at a low doping level (≤3 at% Re) via mixed-valence states Re4+/Re6+ for at least 0.03 < x ≤ 0.10, up to nearly pure Re4+ in V0.70Re0.30O2. Thus, compositions V1−xRexO2 with only one valence state of Re in the material (Re6+ or Re4+) can be obtained as a single phase, while intermediate compositions are subjected to a phase separation, presumably due to different valence states of Re.


 Please wait while we load your content...
Please wait while we load your content...