Location of photocatalytic oxidation processes on anatase titanium dioxide
Abstract
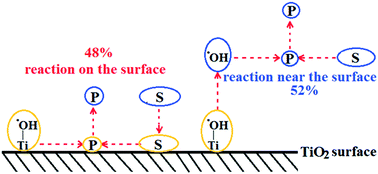
The detailed mechanism of photocatalytic oxidation processes on the TiO2 surface is still not completely clear, particularly the location of degradation processes. There are debates whether photocatalytic reactions happen on the surface or near the surface. To differentiate between processes on the surface and near the surface, we utilized bromate to accelerate the reaction rate and conducted photocatalytic degradation of Rhodamine B with or without pre-adsorption. Since adsorption was slow and the reactions were fast, surface reactions and near-surface reactions could be differentiated. In addition to the experimental data, we conducted theoretical calculations with the Langmuir–Hinshelwood model (surface) and the homogeneous bimolecular reaction model (near surface). Experimental data and theoretical calculations suggested a combination of 47.97% of the surface reaction and 52.03% of the near-surface reaction. This report provides experimental evidence for the location of photocatalytic processes. In addition, through scavenging and the isotope kinetic effect, this report also explored contributions of the conduction band and the valance band, direct hole oxidation, ˙OH radical oxidation, the roles of O2, H2O, and OH−, the impact of pH on the ˙OH formation from the conduction band, and charge carriers' separation. The detailed photocatalytic mechanism was proposed according to the experimental evidence in this report.

 Please wait while we load your content...
Please wait while we load your content...