Reaction of CO, H2O, H2 and CO2 on the clean as well as O, OH and H precovered Fe(100) and Fe(111) surfaces†
Abstract
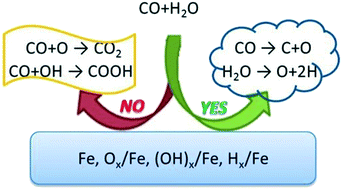
The adsorption and activation of CO, H2O, CO2 and H2 on the clean as well as O, OH and H precovered Fe(100) surface at 0.25 ML coverage and Fe(111) surface at 0.33 ML coverage were computed on the basis of density functional theory (GGA-PBE) to investigate the catalytic activity of metallic iron. On the clean Fe(100) and Fe(111) surfaces, the most favorable reactions are H2O and CO dissociation, and the surface intermediates are the coadsorbed C + O + 2H; CO hydrogenation is kinetically favored and thermodynamically disfavored, whereas CO direct oxidation and COOH formation are kinetically much unfavorable. The O- and OH-precovered Fe(100) surfaces at 0.25 ML coverage and Fe(111) surfaces at 0.33 ML coverage do not promote CO2 formation from the CO direct oxidation and COOH formation from CO and OH coupling, whereas the 0.33 ML O-precovered Fe(111) surface suppresses CO dissociation. In contrast to the 0.25 ML H precovered Fe(110) surface, which does not show a significant effect in the surface reactions, the 0.33 ML H precovered Fe(111) surface can lower the barriers for CO oxidation and hydrogenation, whereas the barrier of COOH formation is increased. It was concluded that the metallic iron surfaces, in particular, the O and OH precovered iron surfaces are not appropriate catalysts for the water-gas shift reaction; however, CO2 dissociation is kinetically and thermodynamically much favorable on all these surfaces. For a given reaction on different surfaces, there is no linear Brønsted–Evans–Polanyi (BEP) correlation between the barriers and reaction energies.


 Please wait while we load your content...
Please wait while we load your content...