Estimation of electric field effects on the adsorption of molecular superoxide species on Au based on density functional theory
Abstract
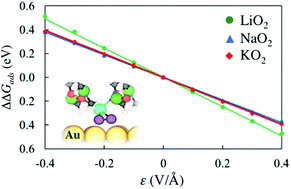
Superoxide species are key intermediates in the oxygen reduction reactions (ORR) that occur at the cathodes of aprotic metal–air batteries. Herein we report a DFT study of the effects of an externally applied electric field (ε) on the stability of various molecular superoxide species, including MO2 (M = Li, Na, K) and O2−, on gold surfaces, which shows that the stability of such species on Au electrodes can be materially affected by the presence of an electric field and solvent molecules, suggesting that such effects should be included in the first-principles modeling of cathode reactions in metal-O2 cells. In the ε range of ±0.4 V Å−1, the stability of MO2 species is found to vary by up to |0.4| eV on Au(111) and |0.2| eV on Au(211) in vacuo, which is larger than the field effects on the stability of O and OH, key intermediates in the ORR by hydrogen. An aprotic solvent such as dimethyl sulfoxide (DMSO), considered here via the inclusion of explicit DMSO molecules above the Au surfaces, stabilizes all three MO2 species at zero fields and amplifies the field effects on the stability of MO2, on both Au surfaces. The variations in the stability of the molecular MO2 species with ε, which have small polarizabilities, are closely approximated by the first-order Stark effect (μ0·ε, where μ0 is the static surface dipole moment induced by adsorption at ε = 0 V Å−1). The superoxide anion (O2−) that has been identified on an under-coordinated Au site has a larger polarizability than the MOx species and a μ0 that is opposite in sign to those of the metal MO2 species, which results in larger errors by the first-order approximation, although its stability varies only moderately under positive electric fields of up to 0.4 V Å−1.


 Please wait while we load your content...
Please wait while we load your content...