NMR probing and visualization of correlated structural fluctuations in intrinsically disordered proteins†
Abstract
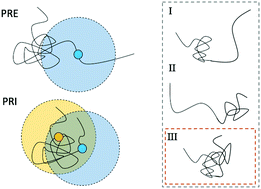
A novel statistical analysis of paramagnetic relaxation enhancement (PRE) and paramagnetic relaxation interference (PRI) based nuclear magnetic resonance (NMR) data is proposed based on the computation of correlation matrices. The technique is demonstrated with an example of the intrinsically disordered proteins (IDPs) osteopontin (OPN) and brain acid soluble protein 1 (BASP1). The correlation analysis visualizes in detail the subtleties of conformational averaging in IDPs and highlights the presence of correlated structural fluctuations of individual sub-domains in IDPs.


 Please wait while we load your content...
Please wait while we load your content...