Spectroscopy of 1,6-diphenyl-1,3,5-hexatriene (DPH) dissolved in three hexane structural isomers, and its consequences on the photophysical model of polyenes
Abstract
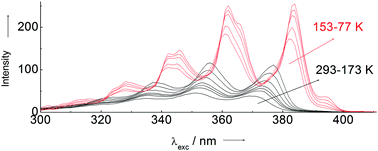
Spectroscopic evidence of the DPH molecules presented in this work allows us to show that the excitation spectrum in n-hexane, obtained by direct immersion in liquid nitrogen, exhibits a peak of origin at 395 nm, with an unexpected intensity, that together with the corresponding peak of origin at 398 nm because of its emission eliminate the abnormal Stokes displacement shown by this compound at room temperature. To the above mentioned explanation we add that the corresponding spectra of DPH dissolved in two structural isomers of n-hexane, 2-methylpentane and 3-methylpentane, do not present these 0–0 transitions (at 395 nm) of DPH. A structural explanation for the peak of origin detected at 395 nm in n-hexane is clear-cut, that is, the experimental evidence totally discards the need to explain the photophysics of the DPH molecules based on the existence of an underlying phantom state (1Ag) as proposed by Hudson and Kohler. This conclusion is strongly supported by monitoring the behavior shown by the DPH spectra obtained by slowly lowering down the temperature of the corresponding solution from 293 to 77 K.


 Please wait while we load your content...
Please wait while we load your content...