Formation of coronene:water complexes: FTIR study in argon matrices and theoretical characterisation†
Abstract
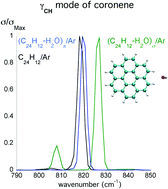
In this paper, we report a combined theoretical and experimental study of coronene:water interactions in low temperature argon matrices. The theoretical calculations were performed using the mixed density functional-based tight binding/force field approach. The results are discussed in the light of experimental matrix isolation FTIR spectroscopic data. We show that, in the solid phase, (C24H12)(H2O)n (n ≤ 6) σ-type complexes, i.e. with water molecules coordinated on the edge of coronene, are formed, whereas in the gas phase, π-interaction is preferred. These σ-complexes are characterised by small shifts in water absorption bands and a larger blue shift of the out-of-plane γ(CH) deformation of coronene, with the shift increasing with the number of complexed water molecules. Such σ interaction is expected to favour photochemical reaction between water and coronene at the edges of the coronene molecule, leading to the formation of oxidation products at low temperature, even in the presence of only a few water molecules and at radiation energies below the ionisation potential of coronene.


 Please wait while we load your content...
Please wait while we load your content...