Molecular and genetic basis for early stage structural diversifications in hapalindole-type alkaloid biogenesis†
Abstract
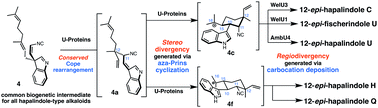
Heterologous expressions and purifications of all WelU proteins from the welwitindolinone pathways in Hapalosiphon welwitschii UTEX B1830 and IC-52-3 led to the discovery that WelU1 and WelU3 selectively assemble 12-epi-fischerindole U (2) and 12-epi-hapalindole C (1), respectively, from 3-geranyl 3-isocyanovinyl indolenine (4) via an enzymatic cascade featuring the Cope rearrangement, stereoselective aza-Prins cyclization and regioselective carbocation deposition. In combination with the in vitro characterization of WelU1/WelU3-homolog AmbU4 for the biogenesis of 12-epi-hapalindole U, this study provide a unified view on the origin of the early stage structural diversifications in hapalindole-type alkaloid biosynthesis, post common intermediate 4.


 Please wait while we load your content...
Please wait while we load your content...