Palladium-catalyzed domino Heck/intermolecular cross-coupling: efficient synthesis of 4-alkylated isoquinoline derivatives†
Abstract
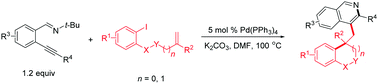
A highly efficient Pd-catalyzed Heck-type cascade process with 2-(1-alkynyl)benzaldimines has been developed, which provides access to a broad range of 4-alkylated isoquinoline derivatives in moderate to good yields. The σ-alkylpalladium(II) intermediate in the Heck reaction activates alkynes toward intramolecular nucleophilic attack. This is the first example of a σ-alkylpalladium(II) intermediate promoting the cyclization of alkynes containing a proximate nucleophilic center.


 Please wait while we load your content...
Please wait while we load your content...