Synthesis of end-functionalized glycopolymers containing α(2,8) disialic acids via π-allyl nickel catalyzed coordinating polymerization and their interaction with Siglec-7†
Abstract
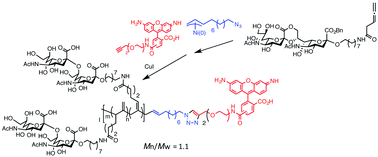
An allene monomer containing an α(2,8) disialic acid was polymerized by a π-allyl nickel complex with an azido group to produce end-functional glycopolymers with an excellent polydispersity index. The polymers allowed terminal modification with a fluorescent dye by click chemistry. The glycopolymers can dissociate Siglec-7–GD3 interactions at low concentrations.


 Please wait while we load your content...
Please wait while we load your content...