Solution processed SnO2:Sb transparent conductive oxide as an alternative to indium tin oxide for applications in organic light emitting diodes
Abstract
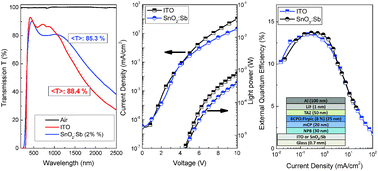
Here, we present the deposition of antimony-doped tin oxide thin films using the ambient spray pyrolysis technique and demonstrate their implementation as transparent electrodes (anodes) in red, green and blue organic light emitting diodes. The films were spray coated at 380 °C from SnCl4 and SbCl3 solution blends in methanol and ∼230 nm thick films were investigated by means of X-ray diffraction, AFM, UV-Vis absorption spectroscopy, 4-point probe, Hall effect measurements and Kelvin probe. It was found that for optimum antimony doping in the precursor solution of ∼2 wt%, the as-deposited ATO films exhibit excellent characteristics such as a low surface roughness (RRMS) of ∼ 6.3 nm, a high work function (∼−5.03 eV), a wide direct band gap (∼4.2 eV), high transparency in the visible spectrum in excess of 85% on glass, a low sheet resistivity (∼32 Ohms sq−1), a high charge carrier concentration (∼6.35 × 1020 cm−3) and a carrier mobility of ∼32 cm2 V−1 s−1. Furthermore, the electrical and optical performance i.e. the turn on voltage and external quantum efficiency of red, green and blue OLEDs fabricated on optimized SnO2:Sb films were identical to those of OLEDs fabricated on commercially available ITO (Rs ∼ 15 Ohms sq−1) and were found to be in excess of 11%, 0.3% and 13% for red, green and blue OLEDs, respectively.

 Please wait while we load your content...
Please wait while we load your content...