Linking coupled motions and entropic effects to the catalytic activity of 2-deoxyribose-5-phosphate aldolase (DERA)†
Abstract
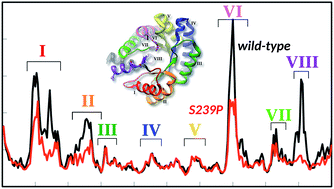
DERA, 2-deoxyribose-5-phosphate aldolase, catalyzes the retro-aldol cleavage of 2-deoxy-ribose-5-phosphate (dR5P) into glyceraldehyde-3-phosphate (G3P) and acetaldehyde in a branch of the pentose phosphate pathway. In addition to the physiological reaction, DERA also catalyzes the reverse addition reaction and, hence, is an interesting candidate for bio-catalysis of carbo-ligation reactions, which are central to synthetic chemistry. An obstacle to overcome for this enzyme to become a truly useful biocatalyst, however, is to relax the very strict dependency of this enzyme on phosphorylated substrates. We have studied herein the role of the non-canonical phosphate-binding site of this enzyme, consisting of Ser238 and Ser239, by site-directed and site-saturation mutagenesis, coupled to kinetic analysis of mutants. In addition, we have performed molecular dynamics simulations on the wild-type and four mutant enzymes, to analyse how mutations at this phosphate-binding site may affect the protein structure and dynamics. Further examination of the S239P mutant revealed that this variant increases the enthalpy change at the transition state, relative to the wild-type enzyme, but concomitant loss in entropy causes an overall relative loss in the TS free energy change. This entropy loss, as measured by the temperature dependence of catalysed rates, was mirrored in both a drastic loss in dynamics of the enzyme, which contributes to phosphate binding, as well as an overall loss in anti-correlated motions distributed over the entire protein. Our combined data suggests that the degree of anticorrelated motions within the DERA structure is coupled to catalytic efficiency in the DERA-catalyzed retro-aldol cleavage reaction, and can be manipulated for engineering purposes.


 Please wait while we load your content...
Please wait while we load your content...