Mechanistic study of allopurinol oxidation using aldehyde oxidase, xanthine oxidase and cytochrome P450 enzymes†
Abstract
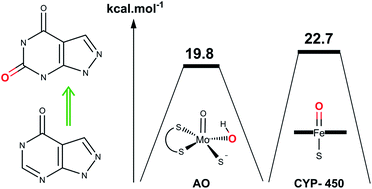
The intrinsic reactivity of allopurinol oxidation is calculated using the active-site models of aldehyde oxidase and xanthine oxidase (molybdopterin cofactor, MoCo) enzymes. The effects of substrate tautomerization, deprotonation and its orientation with respect to the active-site were considered. Among several mechanistic approaches, the most favorable mechanisms are the stepwise pathways. The transition barrier for the most stable tautomer of allopurinol is around 19.83 kcal mol−1. The stepwise approaches indicate the significant role of glutamate → glutamic acid conversion in the catabolism of allopurinol. Both aldehyde oxidase (AO) and xanthine oxidase (XO) could metabolize the allopurinol to oxypurinol through the self-inhibitory mechanism. This pathway is not in accordance with the experimental results which showed the release of oxypurinol with a low half-life. This phenomenon and also the presence of experimental evidence of allopurinol interaction with cytochrome P450 and drugs which are metabolized with P450 encouraged us to investigate allopurinol oxidation using the active species of P450 enzyme, compound I (Cpd I). The P450 enzymes are responsible for the catabolism of the overwhelming majority of drugs. The detailed understanding of the P450-catalyzed mechanism could result in more precise predictions about the drug metabolism and also the drug–drug interactions. The results of this study reveal that cytochrome P450 can successfully metabolize allopurinol with both a thermodynamically and kinetically feasible mechanism. The rate-determining step of this mechanism is around 22.77 kcal mol−1. Allopurinol oxidation with P450 will lead to the release of oxypurinol, not a self-inhibitory mechanism. The outcomes of this investigation can be extended to the other purine or pyrimidine containing compounds (especially hypoxanthine) which are metabolized by this group of enzymes.

 Please wait while we load your content...
Please wait while we load your content...