Expanded investigations of the aglycon promiscuity and catalysis characteristic of flavonol 3-O-rhamnosyltransferase AtUGT78D1 from Arabidopsis thaliana†
Abstract
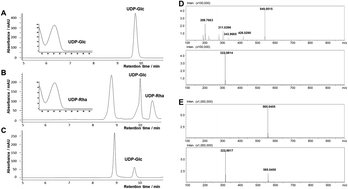
Rhamnosides usually possess better bioavailabilities and improved solubilities compared with their aglycons and are a major source of bioactive natural products. However, biosynthesis of rhamnosides is hindered by the commercially expensive UDP-rhamnose (UDP-Rha) donor and a lack of universal rhamnosyltransferases. In the present study, an efficient UDP-Rha production system via a two-step enzymatic reactions using UDP-glucose (UDP-Glc) as a substrate was constructed. Extensive in vitro enzymatic assays and preparative reactions using the obtained UDP-Rha/UDP-Glc highlighted the robust glycosylation promiscuity of the reported rhamnosyltransferase AtUGT78D1. Based on HPLC-UV and HR-MS analyses, 30 of the tested aromatic compounds belonging to 7 structural types, including flavonoids, flavonoid glycosides, phenylethyl chromones, benzophenones, coumarins, lignanoids, and anthraquinones, were accepted by AtUGT78D1 to conduct the corresponding rhamnosylation and/or glucosylation with one or more glycosyl substitutions at different positions. Further preparative reactions expanded the catalytic characteristic of AtUGT78D1 since it can catalyse the rhamnosylation at the 3-OH position of the flavonols, glucosylation at the 7-OH position of the flavone baicalein, and multiple hydroxyl substitutions for diverse types of aromatics. Interestingly, a unique reversible catalysis activity of AtUGT78D1 was observed, and it has been effectively used in one-pot rhamnosylation of the desired rhamnoside. The enzymatic rhamnosylations of diverse “drug-like” scaffolds as well as bidirectional catalysis for one-pot rhamnosylations by plant rhamnosyltransferase were rarely reported before, which indicated that AtUGT78D1 was expected to be a universal and effective tool for chemo-enzymatic synthesis of diverse bioactive rhamnosylated derivatives for drug discovery.

 Please wait while we load your content...
Please wait while we load your content...