Identification of novel thermostable ω-transaminase and its application for enzymatic synthesis of chiral amines at high temperature†
Abstract
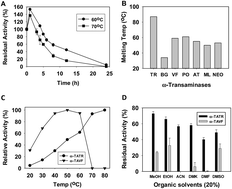
A novel thermostable ω-transaminase from Thermomicrobium roseum which showed broad substrate specificity and high enantioselectivity was identified, expressed and biochemically characterized. The advantage of this enzyme to remove volatile inhibitory by-products was demonstrated by performing asymmetric synthesis and kinetic resolution at high temperature.

 Please wait while we load your content...
Please wait while we load your content...