Bis(diphenylamino)naphthalene host materials: careful selection of the substitution pattern for the design of fully solution-processed triple-layered electroluminescent devices†
Abstract
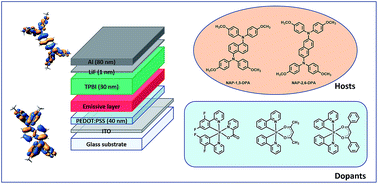
Two new triarylamine-based wide bandgap small molecules differing by the position of their substituents were investigated as hosts for solution-processed organic light-emitting diodes (OLEDs). Blue-green, green and red OLEDs were realized with FIrpic, Ir(ppy)2(acac) and Ir(ppy)2(dbm) as triplet emitters respectively and the three layers constituting the device stacking were successively deposited with orthogonal solvents. Interestingly, one of the two bis(diphenylamino)naphthalene-based compounds, (NAP-1,5-DPA), furnished significantly enhanced EL performances compared to its isomeric counterpart. A maximum luminance of 3905 cd m−2 at 21 V was notably achieved with this material for devices comprising FIrpic as dopant. To get a deeper insight into these major differences in devices, the two host materials were characterized by UV-visible absorption and luminescence spectroscopy, and cyclic voltammetry. Thin films of the two materials were also examined by optical and atomic force microscopy. Thermal properties of the two hosts were also investigated as well as their electronic characteristics by theoretical calculations.

 Please wait while we load your content...
Please wait while we load your content...