Promotional effect of ionic liquids in electrophilic fluorination of methylated uracils†
Abstract
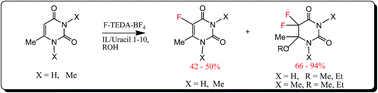
A novel efficient protocol has been developed for fluorination of methylated uracils involving a stoichiometric amount of ionic liquid (IL) in alcohols. The fluorination of 6-methyluracil and 1,3,6-trimethyluracil has been carried out using the electrophilic fluorinating reagent Selectfluor™ (F-TEDA-BF4) in MeOH and EtOH solvents with the formation of 5-fluoro-6-methyluracil, 5-fluoro-1,3,6-trimethyluracil as well as α-fluoromethoxy- and α-fluoroethoxy ethers of difluorodihydrouracils as the main products. The use of a stoichiometric amount of ionic liquid as an additive results in acceleration of the reaction. It has been found that the effect of the anion of the IL on the rate of the reaction is more pronounced compared to that of the cation, the effectiveness of the anions decreasing in the following order: [HSO4−] > [OTf−] ∼ [NTf2−] > [BF4−] > [PF6−]. The influence of metal carbonates on the yields of fluorouracils has also been evaluated.

 Please wait while we load your content...
Please wait while we load your content...