Precisely regulated galactosylation of nucleoside analogues in aqueous hydrophilic solvents catalyzed by solvent-stable β-galactosidase†
Abstract
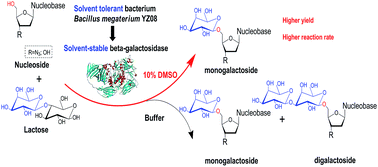
Efficient synthesis of unitary β-galactosyl nucleoside analogues was successfully achieved in a 10% DMSO solvent system, catalyzed by a newly isolated solvent-stable β-galactosidase from solvent-tolerant Bacillus megaterium YZ08. The most efficient sugar donor for the galactosylation of nucleoside analogues was lactose rather than nitrophenyl glycoside. However, it was accompanied with the formation of complicated digalactosyl nucleoside analogues in the buffer system. The addition of DMSO not only prominently increased the stabilization of β-galactosidase but also regulated the products from complicated products to unitary monogalactosyl nucleoside analogues, which could significantly simplify the process of subsequent separation. Strikingly, the affinity of β-galactosidase to 3′-azido-3′-deoxythymidine in 10% DMSO was significantly enhanced, and the catalytic efficiency (kcat/Km) was doubled as compared to that in buffer solution. These results indicated that solvent-stable glycosidase possesses attractive potency in biosynthesis as well as in regulation of the glycoside prodrugs by adjusting the reaction solvent system.

 Please wait while we load your content...
Please wait while we load your content...