Oxidation of CO by N2O over Al- and Ti-doped graphene: a comparative study†
Abstract
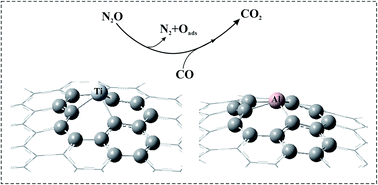
In this work, we employ density functional theory calculations to investigate the CO oxidation mechanisms by N2O molecules over Al- or Ti-doped graphene (Al–/Ti–graphene). The reaction barriers and thermodynamic parameters are calculated using the M06-2X density functional with the 6-31G* basis set. The possible reaction pathway proposed for the oxidation of CO with N2O molecules is as follows: N2O → N2 + Oads and CO + Oads → CO2. Unlike Al–graphene, upon adsorption of the N2O molecules over Ti–graphene, they are quickly dissociated into N2 and Oads species via a barrier-less reaction. Then, the activated Oads reacts with CO molecules to form CO2 molecules. The calculated activation energies of the reaction CO + Oads → CO2 on Al– and Ti–graphene are calculated to be 0.06 and 0.16 eV, which are lower than those on traditional noble metal catalysts. Our results indicate that both Al– and Ti–graphene can be used as a potential catalyst for low-temperature CO oxidation by N2O molecules.

 Please wait while we load your content...
Please wait while we load your content...