Reversible intercalation of aluminium into vanadium pentoxide xerogel for aqueous rechargeable batteries†
Abstract
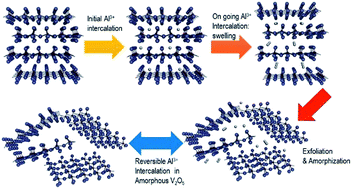
Rechargeable batteries based on the intercalation of aluminium ions may be competitive against lithium-ion batteries, but their development and comprehension are full of difficulties. The charge/discharge processes are particularly complex in aqueous electrolyte solutions. The electrochemical behaviour of orthorhombic V2O5, obtained from xerogel, in an aluminium cell is studied here by using electrochemical cycling, impedance spectroscopy, XRD and XPS results. After electrochemical intercalation of aluminium, the resulting (Al3+)x/3[(V4+)x,(V5+)2−x]O5·nH2O is XRD-amorphous at approximately x = 0.5. The reversible capacity is ca. 120 mA h g−1 (equivalent to Al0.27V2O5). The loss of crystallinity induced by the electrochemical intercalation enhances the chemical exchange between the electrode material and the electrolyte solution. In the presence of acidic water solution, besides the faradic electrochemical process driven by the electrical current, aluminium, protons and water also can be intercalated into V2O5 by chemical reactions or ion exchange.

 Please wait while we load your content...
Please wait while we load your content...