Conformational perturbation, hydrophobic interactions and oligomeric association are responsible for the enhanced chaperone function of Mycobacterium leprae HSP18 under pre-thermal condition
Abstract
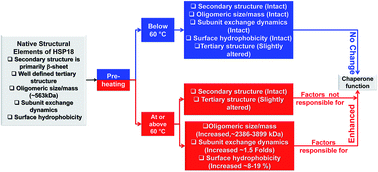
Mycobacterium leprae HSP18 is a small heat shock protein that helps in growth and survival of Mycobacterium leprae pathogen in host species. Recently, we have shown that its chaperone function is enhanced upon heating from 31 to 43 °C which is accompanied by rapid rearrangement of its subunits. We also demonstrated a decrease in its chaperone function when its oligomeric assembly dissociates. However, effect of pre-heating on the structure–function of HSP18 yet remains unexplored. In the present study, we demonstrate that HSP18 undergoes oligomeric association upon pre-heating at 60 °C or above. Surface hydrophobicity and subunit exchange kinetics are also enhanced under similar conditions, which altogether enhances its chaperone function. This study also reveals that perturbation in the secondary structure of HSP18 is completely reversible when heated even at 70 °C. However, alterations in its surface hydrophobicity and quaternary structure do not recover upon pre-heating at 60 °C and above. Interestingly, when pre-heated below 60 °C, conformational (except tertiary conformation) perturbations in HSP18 are completely reversible. Also, its chaperone function remains unaltered when pre-heated below 60 °C. Thus, conformational fluctuations in quaternary structure and subunit exchange dynamics may be the two most important mechanisms by which HSP18 exhibits chaperone function when exposed to such non-permissible thermal stress. Our data reveals that HSP18 is a very robust protein and can recover its native structural integrity as and when the stress disappears. Possibly, this is an important antigen which influences the survival of Mycobacterium leprae pathogen when it encounters thermal stress in an infected host.

 Please wait while we load your content...
Please wait while we load your content...