Dilution or heating induced thickening in a sodium dodecyl sulfate/p-toluidine hydrochloride aqueous solution
Abstract
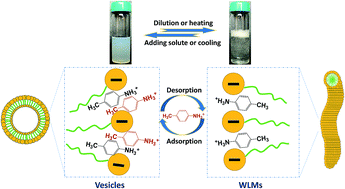
The formation of unilamellar vesicles was successfully established by the addition of hydrotropic salt p-toluidine hydrochloride (PTHC) to solutions of the anionic surfactant sodium dodecyl sulfate (SDS) at high salt concentrations ([PTHC]/[SDS], xPTHC > 0.6). Further studies on the existing vesicles were conducted in terms of changes of concentrations or temperatures of the aqueous solutions. Upon dilution or heating these vesicles can transform into long, flexible wormlike micelles (WLMs). In this process, the solutions switch from an aqueous solution of bluish and nearly Newtonian liquids with low viscosity to clear and viscoelastic solutions with an ability to trap bubbles, which was called “dilution-thickening” or “thermo-thickening”. It should be noted that a microscopic phase separation always emerges at a narrow range of concentrations or temperatures during the transition. Rheological techniques, laser light scattering (LLS), transmission electron microscopy (TEM), micro-differential scanning calorimetry (micro-DSC), and transmittance measurements were used to confirm the formation of vesicles and their reversible transformation with WLMs. Finally, a tentative mechanism for the reversible vesicle-to-WLM transition was proposed to explain the results.

 Please wait while we load your content...
Please wait while we load your content...