Species-specific thiol-disulfide equilibrium constants of ovothiol A and penicillamine with glutathione
Abstract
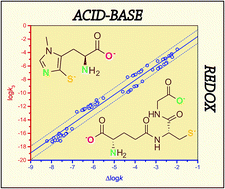
The highly composite acid–base and redox equilibria of thiols can so far be converted into pH-dependent, apparent redox equilibrium constants only. In this work, microscopic redox equilibrium constants were determined to quantify the thiol-disulfide equilibria of biologically important substances in intriguing interactions. The thiol-disulfide redox equilibria of glutathione with ovothiol A and penicillamine were analyzed by quantitative NMR methods to characterize the codependent acid–base and redox equilibria. The directly obtained, pH-dependent, conditional constants were then decomposed to pH-independent, microscopic redox equilibrium constants. These equilibria and the 96 different microscopic redox equilibrium constants are highly influenced by the protonation stage of the adjacent basic moieties. Our data show that equilibrium constants referring to redox systems of covalently identical molecular skeletons, but different acid–base status, can differ by orders of magnitude. In fact, this difference can be as many as 14 orders of magnitude in the glutathione–ovothiol system. This enormous diversity is due primarily to the extreme sensitivity of the ovothiol sulfur to the protonation-evoked electron-withdrawing effects of neighboring basic groups. The thiolate oxidizabilities show close correlation with the respective thiolate basicities and provide sound means for the development of potent agents against oxidative stress.

 Please wait while we load your content...
Please wait while we load your content...