Hydrothermal synthesis of hardened diatomite-based adsorbents with analcime formation for methylene blue adsorption
Abstract
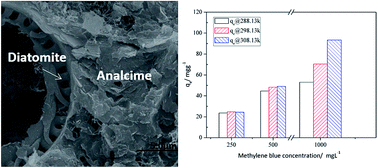
A facile hydrothermal method has been developed to synthesize natural diatomite into hardened diatomite-based adsorbents with zeolite (analcime) formation for methylene blue (MB) adsorption. The results showed that the initial and final strengths were provided with the formed C–S–H gel and zeolite (analcime), respectively. Due to the low temperature synthesis, the formed analcime and retained diatomite were also found to exert a synergistic effect on MB adsorption. The NaOH concentration had a significant effect on the C–S–H and analcime formations, and a lower NaOH concentration (≤9 M) was favorable for C–S–H gel formation, while analcime formed readily at a higher NaOH concentration (≥12 M). The curing temperature and time also influenced the formation of analcime, a long curing time (≥12 h) or a high temperature (≥473 K) was favorable for analcime formation, while an over-long time (≥24 h) or over-high temperature (≥493 K) had a negative effect on the strength of the specimens. The adsorption of MB in this study followed pseudo-second-order kinetics, with a maximum adsorption capacity of 129.87 mg g−1 at 308 K, according to the Langmuir model. Thermodynamic studies also showed that the adsorption process was spontaneous and endothermic. As such, tough diatomite-based adsorbents with analcime formation could be synthesized hydrothermally, and could be used to capture MB in wastewater efficiently.

 Please wait while we load your content...
Please wait while we load your content...