Targeting the colchicine site in tubulin through cyclohexanedione derivatives†
Abstract
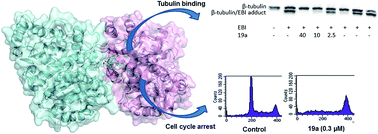
Cyclohexanedione derivatives represent a new family of colchicine-site binders that were identified through a ligand-based virtual screening approach. Structural modifications have now been performed at both distal sites of our identified hit [2-(1-((2-methoxyphenyl)amino)ethylidene)-5-phenylcyclohexane-1,3-dione (4)] in order to improve tubulin binding affinity, anti-proliferative activity and/or aqueous solubility. The results obtained indicate that the 2-methoxyphenyl ring, the fragment located closer to the αβ-tubulin interface according to docking studies, is the one that allows structural variation in order to improve the Ka value against tubulin (as in compound 20a with a Ka = 1.3 × 107 M−1, analogous to colchicine) or to improve aqueous solubility, as in compound 22c, being more than 10 times more soluble than the previous hit 4.


 Please wait while we load your content...
Please wait while we load your content...