Efforts to total synthesis of philinopside E: convergent synthesis of the sulfated lanostane-type tetraglycoside†
Abstract
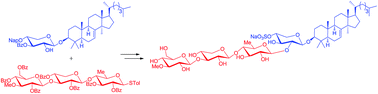
As an important step to total synthesis of philinopside E with important antitumor activities (Ed50 0.75–3.50 μg mL−1), we described herein convergent synthesis of a triterpene glycoside composed of the sulfated tetrasaccharide residue identical to that of philinopside E and the aglycone of lanost-7-en-3β-ol. The stereocontrolled synthesis of the aglycone from 24,25-dihydrolanosterol was accomplished relying on the stereoselective reductions of the 2,3-unsaturated-1,4-diketone system assisted by a C3-tert-butyldimethylsilyloxy group and convenient installation of the required 7(8)-double bond via syn elimination of triflate. Sequencial convergent coupling of monoglycoside, prepared by reacting the aglycone with orthogonally protected xylosyl thioglycoside, with trisaccharide thioglycoside originated from glucose, xylose and quinovose derivatives, incorporation of sulfation and deprotection afforded the target molecule. The features of our work are that the four 1,2-trans glycosidic bonds were stereoselectively constructed and the precious aglycone was introduced in the late-stage synthesis, which would facilitate the total synthesis of philinopside E and related natural products.

 Please wait while we load your content...
Please wait while we load your content...