Abstract
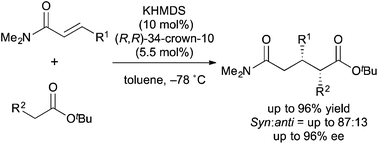
In the presence of catalytic amounts of potassium hexamethyldisilazide (KHMDS) and a chiral macrocyclic crown ether, asymmetric 1,4-addition reactions of simple esters with α,β-unsaturated amides proceeded to afford the desired 1,4-adducts in high yields with good stereoselectivities. Mechanistic investigations revealed that proper combination of substrates was a key for the efficient catalytic turnover.
- This article is part of the themed collections: HOT articles in Organic Chemistry Frontiers in 2016 and Celebrating the 75th Birthday of Professor Barry Trost

 Please wait while we load your content...
Please wait while we load your content...