Palladium-catalyzed alkyne polyannulation of diphenols and unactivated internal diynes: a new synthetic route to functional heterocyclic polymers†
Abstract
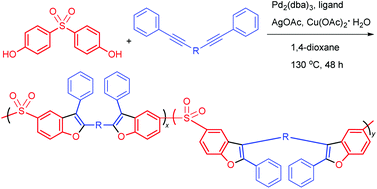
A new polymerization route for the preparation of functional heterocyclic polymers was developed from diphenol derivatives and unactivated internal diynes. The alkyne polyannulations of 4,4′-sulfonyldiphenol and 4,4′-(α,ω-alkylenedioxy)bis(diphenylacetylene)s or 1,2-bis[4-(2-phenylethynyl)phenyl]-1,2-diphenylethene were catalyzed by Pd2(dba)3 in the presence of bathophenanthroline, silver acetate and copper(II) acetate monohydrate in 1,4-dioxane at 130 °C, affording polymers with benzofuran moieties in satisfactory yields and high molecular weights (Mw up to 34 000). All the polymers were thermally stable, losing merely 5% of their weight at high temperatures of up to 376 °C. They showed a good film-forming ability and their thin solid films showed high refractive indices (RI = 1.900–1.611) in a wide wavelength region of 400–1000 nm. The polymer carrying tetraphenylethene units in the backbone was photosensitive and could be utilized to generate a fluorescent pattern by the photolithography process.

 Please wait while we load your content...
Please wait while we load your content...