Ionic hydrogen bond donor organocatalyst for fast living ring-opening polymerization†
Abstract
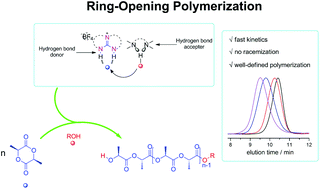
H-bonding organocatalysis using (thio)urea and amine has been very successful. Ionic H-bonding catalysis, especially in polymerizations, has scarcely been explored. Here we disclose guanidinium hexahydro-2H-pyrimido[1,2-a]pyrimidin-1-ium [(HppH2)+] as a representative ionic H-bond donor (IHBD), combined with tertiary amines as H-bond acceptors (HBA), promoted fast ring-opening polymerization (ROP) of L-lactide (LLA). The positively charged IHBD guanidinium exhibits exceptional activating ability towards monomer LLA. Its partnership with (−)-sparteine demonstrates excellent IHBD–HBA binary catalysis in the ROP of LLA, which featured conversions up to 97%, predicted molecular weights (from 2550 to 17 900 g mol−1), narrow dispersities (Đ ≤ 1.12), and short reaction times of 30 to 180 minutes. A bifunctional synergistic activation mechanism by (HppH2)+ and (−)-sparteine is proposed, and is supported by NMR measurements. The controlled/living nature of the ROP is confirmed by kinetics and chain extension experiments. 1H NMR, SEC, and MALDI-ToF MS analyses strongly indicate that the obtained poly(L-lactide)s were exactly the designated ones. The successful synthesis of well-defined poly(trimethylene carbonate)-block-poly(L-lactide) verifies that the catalytic ROPs were of a controlled/living nature, and suggests that the IHBD–HBA binary catalysis system is generally applicable.


 Please wait while we load your content...
Please wait while we load your content...