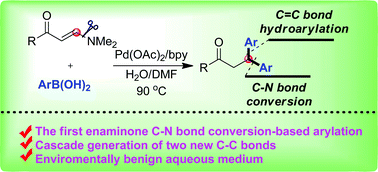
Synthesis of β,β-diaryl propiophenones via palladium-catalyzed domino arylboronation, elimination and enone hydroarylation of enaminones†
Abstract
The syntheses of β,β-diaryl aryl propiophenones have been realized via palladium-catalyzed domino reactions of dimethyl amino functionalized enaminones and aryl boronic acids. This is the first example of transition metal-catalyzed enaminone C–N bond conversion for the generation of a new C–C(aryl) structure.
 Please wait while we load your content...
Please wait while we load your content...