Chemoselective modifications for the traceless ligation of thioamide-containing peptides and proteins†
Abstract
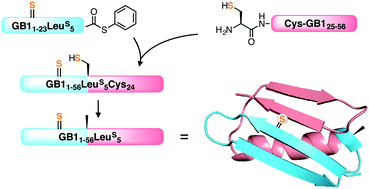
Thioamides are single-atom substitutions of canonical amide bonds, and have been proven to be versatile and minimally perturbing probes in protein folding studies. Previously, our group showed that thioamides can be incorporated into proteins by native chemical ligation (NCL) with Cys as a ligation handle. In this study, we report the expansion of this strategy into non-Cys ligation sites, utilizing radical initiated desulfurization to “erase” the side chain thiol after ligation. The reaction exhibited high chemoselectivity against thioamides, which can be further enhanced with thioacetamide as a sacrificial scavenger. As a proof-of-concept example, we demonstrated the incorporation of a thioamide probe into a 56 amino acid protein, the B1 domain of Protein G (GB1). Finally, we showed that the method can be extended to β-thiol amino acid analogs and selenocysteine.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins
 Please wait while we load your content...
Please wait while we load your content...