Iridium(iii)-catalyzed regioselective C7-sulfonamidation of indoles†
Abstract
Iridium(III)-catalyzed direct C7-sulfonamidation of indoles with sulfonyl azides is described. The developed method has good compatibility with diverse functional groups, providing various 7-amino-substituted indoles with good to excellent yields in a short time under mild reaction conditions. The key feature of the developed method is the regioselective functionalization at the C7-position of 2,3-unsubstituted indoles. Biologically active compounds can be obtained using this protocol. The application of the iridium(III) catalyst and directing group plays a crucial role in the regioselectivity of the developed reaction.
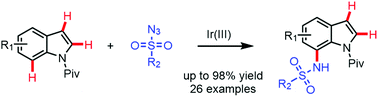
 Please wait while we load your content...
Please wait while we load your content...