From solution to in-cell study of the chemical reactivity of acid sensitive functional groups: a rational approach towards improved cleavable linkers for biospecific endosomal release†
Abstract
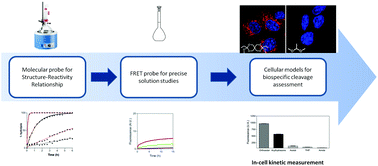
pH-Sensitive linkers designed to undergo selective hydrolysis at acidic pH compared to physiological pH can be used for the selective release of therapeutics at their site of action. In this paper, the hydrolytic cleavage of a wide variety of molecular structures that have been reported for their use in pH-sensitive delivery systems was examined. A wide variety of hydrolytic stability profiles were found among the panel of tested chemical functionalities. Even within a structural family, a slight modification of the substitution pattern has an unsuspected outcome on the hydrolysis stability. This work led us to establish a first classification of these groups based on their reactivities at pH 5.5 and their relative hydrolysis at pH 5.5 vs. pH 7.4. From this classification, four representative chemical functions were selected and studied in-vitro. The results revealed that only the most reactive functions underwent significant lysosomal cleavage, according to flow cytometry measurements. These last results question the acid-based mechanism of action of known drug release systems and advocate for the importance of an in-depth structure-reactivity study, using a tailored methodology, for the rational design and development of bio-responsive linkers.
 Please wait while we load your content...
Please wait while we load your content...