Reagents for diverse iodosilane-mediated transformations†
Abstract
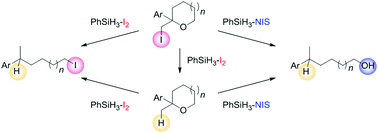
It was observed that a PhSiH2I-mediated protocol using PhSiH3 and cat. I2 caused the deiodination of 2-(iodomethyl)-2-phenyltetrahydrofuran. Stemming from the investigation of the mechanism, we found that the PhSiH3–I2 system selectively promotes diverse cascade transformations from cyclic ethers to acyclic alkyl iodides, and the PhSiH3−N-iodosuccinimide (NIS) system also promotes cascade transformations from cyclic ethers to acyclic alcohols.

 Please wait while we load your content...
Please wait while we load your content...