Organocatalytic asymmetric Michael addition of α-alkylidene succinimides to nitrostyrenes†
Abstract
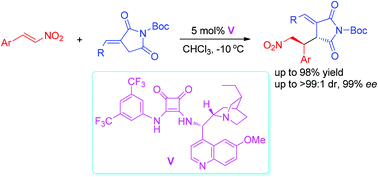
A bifunctional squaramide-catalyzed asymmetric Michael addition reaction of α-alkylidene succinimides with nitrostyrenes and a nitrodiene has been developed. This organocatalytic asymmetric reaction provides easy access to functionalized succinimides with two contiguous stereocenters with a broad substrate scope. The desired succinimide derivatives were obtained in good to excellent yields (up to 98%) with high to excellent diastereoselectivities (up to >99 : 1 dr) and excellent enantioselectivities (up to 99% ee). This protocol provides a straightforward entry to functionalized chiral succinimide derivatives from simple starting materials.
 Please wait while we load your content...
Please wait while we load your content...