Exploring the divalent effect in fucosidase inhibition with stereoisomeric pyrrolidine dimers†
Abstract
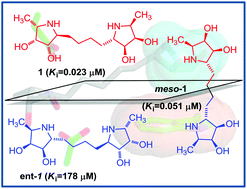
Multi-valent inhibitors offer promise for the enhancement of therapeutic compounds across a range of chemical and biological processes. Here, a significant increase in enzyme-inhibition potencies was observed with a dimeric iminosugar-templated fucosidase inhibitor (IC50 = 0.108 μM) when compared to its monovalent equivalent (IC50 = 2.0 μM). Such a gain in binding is often attributed to a “multivalent effect” rising from alternative recapture of the scaffolded binding epitopes. The use of control molecules such as the meso analogue (IC50 = 0.365 μM) or the enantiomer (IC50 = 569 μM), as well as structural analysis of the fucosidase-inhibitor complex, allowed a detailed analysis of the possible mechanism of action, at the molecular level. Here, the enhanced binding affinity of the dimer over the monomer can be attributed to additional interactions in non-catalytic sites as also revealed in the 3-D structure of a bacterial fucosidase inhibitor complex.

 Please wait while we load your content...
Please wait while we load your content...