Cation–halide transport through peptide pores containing aminopicolinic acid†
Abstract
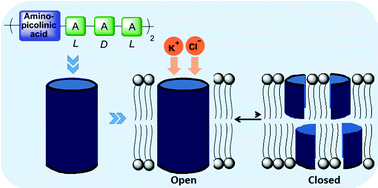
Synthetic pores that selectively transport ions of biological significance through membranes could be potentially used in medical diagnostics or therapeutics. Herein, we report cation-selective octapeptide pores derived from alanine and aminopicolinic acid. The ion transport mechanism through the pores has been established to be a cation–chloride symport. The cation–chloride co-transport is biologically essential for the efficient functioning of the central nervous system and has been implicated in diseases such as epilepsy. The pores formed in synthetic lipid bilayers do not exhibit any closing events. The ease of synthesis as well as infinite lifetimes of these pores provides scope for modifying their transport behaviour to develop sensors.

 Please wait while we load your content...
Please wait while we load your content...