A fragment merging approach towards the development of small molecule inhibitors of Mycobacterium tuberculosis EthR for use as ethionamide boosters†
Abstract
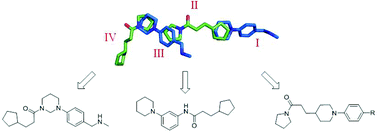
With the ever-increasing instances of resistance to frontline TB drugs there is the need to develop novel strategies to fight the worldwide TB epidemic. Boosting the effect of the existing second-line antibiotic ethionamide by inhibiting the mycobacterial transcriptional repressor protein EthR is an attractive therapeutic strategy. Herein we report the use of a fragment based drug discovery approach for the structure-guided systematic merging of two fragment molecules, each binding twice to the hydrophobic cavity of EthR from M. tuberculosis. These together fill the entire binding pocket of EthR. We elaborated these fragment hits and developed small molecule inhibitors which have a 100-fold improvement of potency in vitro over the initial fragments.
- This article is part of the themed collection: In memory of Chris Abell

 Please wait while we load your content...
Please wait while we load your content...