Multicomponent reactions of phosphines, enynedioates and benzylidene malononitriles generated highly substituted cyclopentenes through an unexpected phosphine α-addition-δ-evolvement of an anion pathway†
Abstract
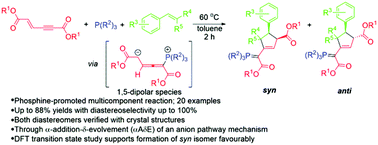
Multicomponent reactions of phosphines, enynedioates and benzylidene malononitriles provide highly substituted syn-selective cyclopentenes appending the phosphorus ylide moiety in good yield with a diastereoselectivity of up to 100% through resonance-derived 1,5-dipolar species as the key intermediates, via the nucleophilic α(δ′)-attack of phosphines toward enynedioates followed by addition to benzylidene malononitriles and 5-exo-dig cyclization. Through computational analyses, the overall reactions for the formation of syn- and anti-diastereomers are both exothermic with 65.6 and 66.3 kcal mol−1 at the B3LYP-D3/6-31G(d) level of theory and were found to be kinetically controlled, which favours the formation of syn-diastereomers.

 Please wait while we load your content...
Please wait while we load your content...