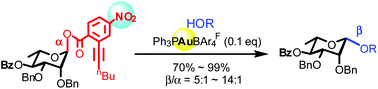
Stereoselective synthesis of β-rhamnopyranosides via gold(i)-catalyzed glycosylation with 2-alkynyl-4-nitro-benzoate donors†
Abstract
Stereoselective β-rhamnopyranosylation remains a challenge, due to the unfavorable anomeric effect and steric hindrance of the C2-substituent; herein, this challenge is addressed with a gold(I)-catalyzed SN2-like glycosylation protocol employing α-rhamnopyranosyl 2-alkynyl-4-nitro-benzoates as donors.

 Please wait while we load your content...
Please wait while we load your content...