Coordination of o-benzosemiquinonate, o-iminobenzosemiquinonate, 4,4′-di-tert-butyl-2,2′-bipyridine and 1,10-phenanthroline anion radicals to oxidovanadium(iv)†
Abstract
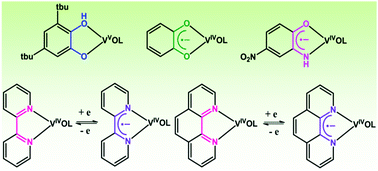
This article reports on the stabilization of organic radical anions promoted by the oxidovanadium(IV) ion. A 3,5-di-tert-butylcatecholate (tBucatH−) complex of oxidovanadium(V) of the type [(LaONO2−)(VO3+)(tBucatH−)] (1) was isolated using tridentate (E)-N′-((3-hydroxynaphthalen-2-yl)methylene)benzohydrazide (LaONOH2) as a coligand, whereas o-benzosemiquinonate (sq˙−) and p-nitro-o-iminobenzosemiquinonate (NO2isq˙−) radical anion complexes of oxidovanadium(IV) of the types [(LNNO−)(VO2+)(sq˙−)] (2) and [(LNNO−)(VO2+)(NO2isq˙−)] (3) were successfully isolated using (1Z,N′E)-N′-(phenyl(pyridin-2-yl)methylene)benzohydrazonic acid (LNNOH) as a coligand. Oxidovanadium(IV) complexes of the types [(LbONO2−)(VO2+)(phen)] (4) and [(LbONO2−)(VO2+)(tBubpy)] (5), which undergo reversible reduction to the 4,4′-di-tert-butyl-2,2′-bipyridine radical anion (tBubpy˙−) and the 1,10-phenanthroline radical anion (phen˙−) to afford the coupled states [(LbONO2−)(VO2+)(phen˙−)]− (4−) and [(LbONO2−)(VO2+)(tBubpy˙−)]− (5−), respectively, were isolated (LbONOH2 = (E)-N′-(2-hydroxybenzylidene)benzohydrazide). The molecular geometries of the complexes were confirmed by the single-crystal X-ray structure determinations of 1, 3 and 4. In 1, the V–Ophenolato length cis to V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is 1.879(2) Å and the dissimilar V–O and V–OH lengths corresponding to the tBucatH− ligand are 1.832(2) and 2.312(2) Å, respectively. In 1, the average C–O lengths in tBucatH− are 1.351(3) Å, whereas in 3 the average C–O and C–N lengths in NO2isq˙− are 1.293(4) and 1.355(5) Å, respectively. In 4, the V–Ophenolato length cis to V
O is 1.879(2) Å and the dissimilar V–O and V–OH lengths corresponding to the tBucatH− ligand are 1.832(2) and 2.312(2) Å, respectively. In 1, the average C–O lengths in tBucatH− are 1.351(3) Å, whereas in 3 the average C–O and C–N lengths in NO2isq˙− are 1.293(4) and 1.355(5) Å, respectively. In 4, the V–Ophenolato length cis to V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1.937(3) Å) is relatively longer. The 51V NMR spectrum of 1 displays a signal at −337.2 ppm, whereas the signals for 2 and 3 are deshielded to +382.4 and +71.8 ppm, respectively. The closed-shell singlet (CSS) solutions of 3 and 5− at the B3LYP/DFT level are unstable and the open-shell singlet (OSS) solutions are 0.5 and 7.3 kcal mol−1 lower in energy, respectively, than the CSS solutions. In 3 and 5− the alpha spin (100%) is localized on the vanadium ion, whereas the beta spin is delocalized across the aminophenol and bipyridine fragments. 2 and 3 exhibit lower-energy absorption bands at 785 and 585 nm, which are defined as CSS → OSS perturbation transitions.
O (1.937(3) Å) is relatively longer. The 51V NMR spectrum of 1 displays a signal at −337.2 ppm, whereas the signals for 2 and 3 are deshielded to +382.4 and +71.8 ppm, respectively. The closed-shell singlet (CSS) solutions of 3 and 5− at the B3LYP/DFT level are unstable and the open-shell singlet (OSS) solutions are 0.5 and 7.3 kcal mol−1 lower in energy, respectively, than the CSS solutions. In 3 and 5− the alpha spin (100%) is localized on the vanadium ion, whereas the beta spin is delocalized across the aminophenol and bipyridine fragments. 2 and 3 exhibit lower-energy absorption bands at 785 and 585 nm, which are defined as CSS → OSS perturbation transitions.

 Please wait while we load your content...
Please wait while we load your content...