Various structures of complexes fabricated using transition metals and triazole ligands and their inhibition effects on xanthine luminescence†
Abstract
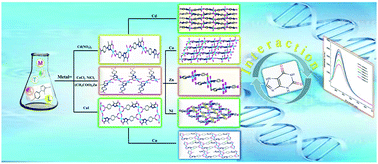
Self-assembled mono- and bi-nuclear complexes of Cd(II) (1), Co(II) (2), Zn(II) (3), Ni(II) (4) and Cu(II) (5) were synthesized via the reaction of 5-methyl-1-(4-carboxylphenyl)-1H-1,2,3-triazole-4-carboxylic acid (H2L) and the corresponding metal salt under hydrothermal conditions. All complexes were characterized using elemental analysis, IR spectroscopy, UV-vis spectroscopy, PXRD analysis, thermogravimetry and single-crystal X-ray diffraction. Structural analyses show that the H2L ligand exhibits diverse coordination modes: μ2-η1O-η1O-η1O-η1N for 1, μ2-η1O-η1O-η1N for 2–4 and μ3-η1O-η1O-η1N-η1O for 5. Based on the various structures and the luminescent response of xanthine (3,7-dihydro-purine-2,6-dione), a new experiment for exploring the interaction between transition metal complexes and luminescent xanthine was performed for the first time. The results show that the luminescence response of xanthine depends on the concentration of metal complex.

 Please wait while we load your content...
Please wait while we load your content...