Reactive extraction of gallic acid with tri-n-caprylylamine
Abstract
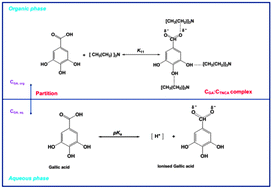
This paper presents an optimization study of reactive extraction of gallic acid from aqueous solution with tri-n-caprylylamine in hexanol. Extraction efficiency was optimized as a function of process variables: initial gallic acid concentration in the aqueous phase (CGA), tri-n-caprylylamine concentration (CTNCA) used as an extractant, and extraction temperature (T). Response surface methodology in conjunction with central composite design containing sixteen experimental runs was statistically employed for the reactive extraction of gallic acid. A statistical model predicted an extraction efficiency of 80.2% at the optimal values of process parameters as follows: CGA = 0.0147 mol L−1, CTNCA = 0.234 mol L−1, and T = 25 °C. Using these optimal parameters under experimental conditions in three independent replicates an extraction efficiency of 81.2% was obtained which was in close agreement with the predicted one.

 Please wait while we load your content...
Please wait while we load your content...